Certification that the cleanroom is operating within the ranges set forth is the final step before occupancy and use of the pharmacy. The goal of the HVAC systems design is so that the users have a functional system that meets their requirements which they have verified is safe for compounding. United States Pharmacopeia (USP) <797> and <800> discuss the parameters design parameters and the requirement for verification every six (6) months but not the procedure for certification. As discussed in the previous article the temperature, humidity, and pressure alarm points should be discussed and established with the user, allowing the certifier to verify those values before occupancy.
The Controlled Environmental Testing Association’s (CETA) mission is to use the criteria from the most current USP <797> and <800>, which is officially being released November 1, 2023, to create guidelines for the procedures for certification of sterile compounding cleanrooms. The cleanrooms are comprised of two parts as defined by USP <797> and <800>, Primary Engineering Controls (PEC) and the Secondary Engineering Controls (SEC), and both need to be certified. The PEC is the fume hood in which the compounding is performed, and the SEC is the cleanroom space in which the PEC is housed. The certifications by CETA are similar for both, with a few exceptions.
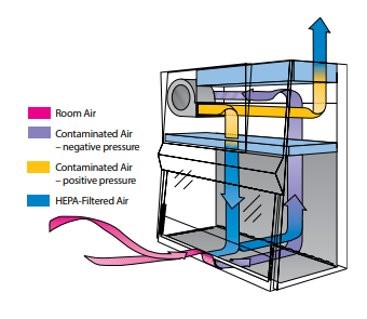
The PEC certification includes a visual test or smoke test to verify that the airflow at the opening to the BSC or fume hood is not too turbulent to draw the fumes out away from the hood, which could cause a hazard for the user compounding the drugs. To help combat this issue, laminar flow diffusers with HEPA filters are recommended for use in the ceiling, and placement is critical to avoid drafts at the PEC openings.
The certification of the SEC has several required tests and a few optional tests. Verifying the airflow quantity into and out of the cleanroom is required; measuring the airflow directly rather than calculating it based on the velocity is recommended. USP <797> and <800> provide minimum air changes per hour (ACH) for each type of space based on the ISO Classification.
Buffer rooms must be ISO Class 7 air quality or better. The actual compounding must be prepared in an ISO Class 5 or better PEC. A PEC for hazardous drug compounding is a Biological Safety Cabinet (BSC) or a fume hood, with an exhaust connection, a recirculating fan, and a HEPA filter to increase clean air circulation. A PEC for non-hazardous drug compounding is a laminar flow hood or a fume hood that recirculates the room air after filtering it through a HEPA filter. Ante rooms that serve as the access and aid control of the air shall be at least ISO Class 8. However, if the anteroom is access for a negative pressure buffer room, such as compounding hazardous drugs, it shall be at least ISO class 7. To verify the ISO Class of the SEC, the CETA certification guide provides parameters for measuring the airborne total particle count. For reference, an ISO Class 8 space requires less than 3,520,000 particle counts per meter cubed of particles less than 0.5 microns measured under dynamic conditions, and USP <797> requires an air exchange rate of more than 20 ACH. A better air quality space, such as an ISO Class 7, must have less than 352,000 particles per meter cubed with more than 30 ACH supply air. While negative pressure rooms have greater exhaust flow rates, USP <797> is focused on the air change rates being sufficient to dilute and remove airborne contaminants and thus consider supply air, not exhaust or return, to ensure dilution.
Room pressurization verification is also a required test, and the pressure gauges installed are required to be verified for accuracy with each certification. CETA discusses the procedure for the tests and the accuracy and resolution of the calibrated manometer required to be utilized. Along with pressure, the temperature and relative humidity are required to be verified to be within the ranges listed and meet the owner’s requirements, as well as verify the sensor accuracy every 12 months. As discussed in the previous article, microbial air and surface sampling should occur if the space is outside of designated parameters for an extended period. The last required test is verifying that there is no supply air leakage around the HEPA filters in the laminar flow diffusers. CETA lists the procedure to use an aerosol medium and an aerosol photometer for testing the filters but cautions that if the room has smoke detectors, disable them first so there are no false alarms.
For newly constructed or renovated cleanrooms, CETA recommends these optional tests for the lighting to verify an acceptable level with the equipment in the room and to test that the noise level is not objectionable. They also list as an option to do a room recovery rate test for troubleshooting by introducing particles and measuring how long the SEC takes to return to a steady state of cleanliness. They recommend this so that the users know how long of an outage to expect when an issue should arise, and the room needs to be cleaned.
Certification is a part of the final closeout when a pharmacy is under construction. Other closeout items related to the HVAC system are the testing, adjusting, and balancing (TAB) and the equipment commissioning. Each of these items takes time and is challenging to perform concurrently; if you are scheduling a closeout of the construction of a pharmacy, be sure to leave time at the end of the project for the systems to be tested and set up properly. This process typically takes at least a month, but can extend longer depending on how well your team works together. If you need more information about the certification process or have a compound pharmacy you are considering renovating, contact Douglas Barnhart, PE, at dcb@ba-inc.com or 717-845-7654 to discuss, and we can share some of the lessons learned from the dozens of other pharmacies we have designed.